FRAUD IN THE DEVELOPMENT OF LUPRON?
by David Redwine (reprinted)
(Lupron is the main puberty blocker used in precocious puberty and gender affirming care-transgendered kids)
Here is the core of the issue: during early (1980's) development of Lupron for the treatment of endometriosis, TAP (Takeda/Abbott Pharmaceuticals) developed information that by one year after stopping Lupron, ovarian function did not return to baseline in most (63%) of the few patients followed that long. This apparent damage to ovarian function long after Lupron was discontinued was hidden from physicians and patients and was revisited by the manufacturer only one time using fabricated evidence. Fast-forward to a 2018 medical publication which reported that 45% of patients who took Lupron as teenagers and who answered a questionnaire 4 or more years after stopping Lupron complained of symptoms suggestive of ovarian dysfunction that they considered to be irreversible and due to Lupron. This is consistent with what the manufacturer knew but kept hidden. How could this later paper not be related to the hidden findings regarding ovarian dysfunction identified over 30 years ago? This means that no patient has ever received proper informed consent before agreeing to Lupron therapy.
From the in-house studies that brought Lupron to market we know several things:
1. Lupron lowers estradiol levels to the menopausal range during therapy (no surprise since that is the goal).
The question becomes: when does the estradiol level return to the pre-treatment baseline level? These are young (18 - 35) patients taking it and they need good ovarian function to return after treatment for health and pregnancy. There is very scant information about this extremely important question although the manufacturer implies that good ovarian function returns in most by 4 - 6 months after stopping Lupron.
2. In study M92-878, estradiol was checked after the first menstrual flow following discontinuation of Lupron, which usually occurred 2 - 3 months after stopping Lupron. So estradiol would have been checked most typically 4 - 6 months after stopping Lupron. This was checked a single time as a 'safety' measure. But a sizable 39% of patients had not had estradiol levels return to baseline. This is even more notable because the baseline estradiol among these patients was already fairly low but was pushed even lower after Lupron. What does that mean?
Assuming without evidence that both the baseline flow and first menstrual flow after stopping Lupron was the result of an ovulatory event, we can apply basic physiology to gain insight into the actions of Lupron vs the human body. If both the baseline and followup estradiol levels were drawn on cycle day 3 -5, that is when FSH, LH, estradiol and progesterone are low. It's a temporary state of slumber. Lupron acts on this quiet-time physiologic combination of pituitary and ovarian functions. Lupron is far more powerful than FSH. When FSH end estradiol are already near their lowest levels of the cycle and Mother Nature can not fall much lower unless with menopause, Lupron is able to suppress the production of estradiol even further as shown by these study results. After pinning the hypothalamic/pituitary/ovarian axis to its back at the most vulnerable time of the cycle, when does Lupron relax its grip on this 39%?
So while it is correct to state that most patients had a return of estradiol to baseline, 39% is a large fraction of patients without a return to baseline estradiol and this % was not mentioned in the journal publication coming out of this study. See first chart below which shows the Lupron-only arm. For simplicity's sake, I didn't show the charts for 3 other arms of the study in which add-back therapies with norethindrone and Premarin were used. However, these 3 other arms are represented on the other graphs below and had very similar results to the Lupron-only group.
Among these patients whose estradiol had not returned to baseline, estradiol declines compared to baseline ranged up (or would it be more correct to say 'down'?) to 90%! This was touted as a 'safety' measure but the estradiol declines were not mentioned in the medical literature publication coming out of this study. Why is it 'safe' that estradiol levels remain lower than baseline in so many patients? Answer: it is not 'safe'. Did the manufacturer already have evidence that estradiol remains depressed long after stopping Lupron and that's why only a single estradiol level was to be checked? The protocol called for only a single estradiol determination so the question remains: when does the estradiol level return to baseline? Maybe later? Is the decline in estradiol seen in M92-878 an aberration for that 39% and they returned to normal or is it the beginning of a trend? Those patients were not examined further, but an earlier study had already provided answers at one year after stopping Lupron. See #3. The medical journal publication coming out of M92-878 was Obstet Gynecol 1998;91:16-24.
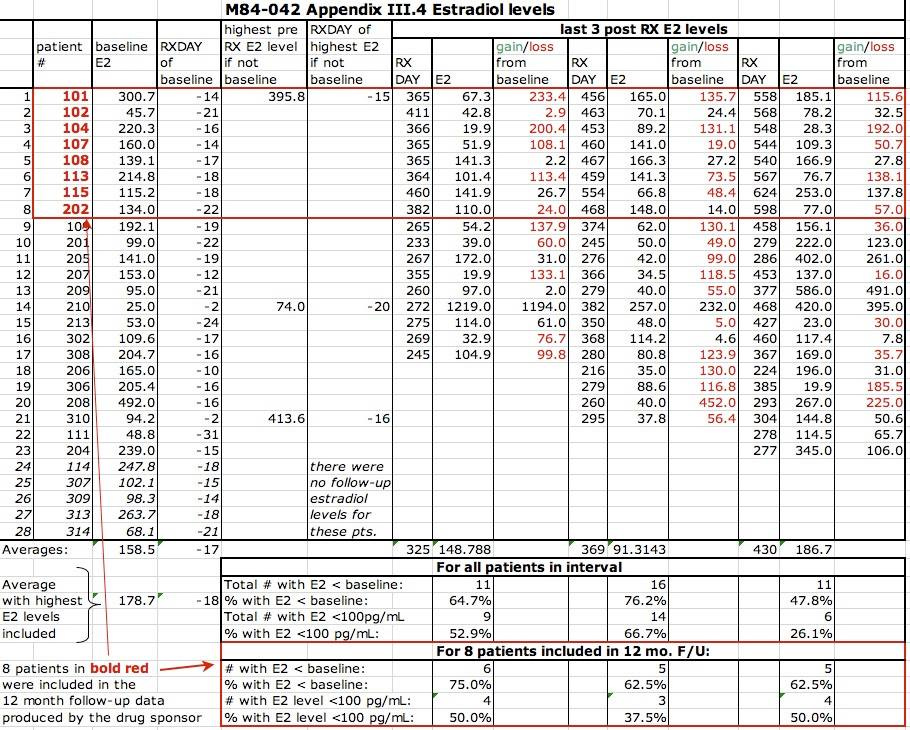
3. Study M84-042 hints at an answer to the question "After stopping Lupron, what happens long-term to ovarian function as reflected by estradiol levels?". In this study, estradiol levels were checked in 8 patients at one year after stopping Lupron. Ignoring for a moment that 8 patients is a very small number, and ignoring that intranasal Lupron was the primary treatment, the estradiol levels at ONE YEAR after stopping Lupron were lower than baseline in 6/8 (75%) of patients, with declines ranging up to 87%. 5/8 (63%) of patients had estradiol levels below 100 pg/mL which is important if it were to stay that low because in a normal ovarian hormonal cycle, the peak levels of estradiol range between 100 and 300 pg/mL. One patient had a followup estradiol level of 28 which is essentially menopausal. See second chart below. One patient (patient 102) had a baseline estradiol of 45.7 and a higher level at one year after stopping Lupron, so seems to have had her estradiol level checked at a different time of the cycle. If that patient is disregarded as one orange being compared to 7 apples, then 6/7 (86%) of patients did not have their estradiol regain baseline levels by one year after stopping Lupron. These 8 patients in that red box in the chart constitute the only evidence that estrogen levels were checked by the manufacturer long after Lupron was discontinued that I found in my review of thousands of pages of in-house studies. 8 patients hidden deep in the data but not discussed in the medical journal publication coming out of that in-house study. 8 patients with objective laboratory evidence suggesting long-term ovarian dysfunction in 39% of patients exposed to the drug. 8 patients whose estradiol decline should have been a sign to get more information on what happens to ovarian function long after Lupron. Unsurprisingly, ovulation also seemed damaged by 1 year after stopping Lupron: of the same 8 patients having follow-up progesterone levels at one year after stopping Lupron, 6 (75%) had 1 year progesterone levels lower than baseline and 5 (62.5%) of these levels were compatible with anovulation (< 1.1). I suspect that there is more evidence about estradiol that was not produced in discovery during Klein v Abbott. See 4 below. The medical journal publication coming out of M84-042 was Fertil Steril 1989;51:390 and contains charts which are not supported by the raw data.
4. Study M86-050 checked estradiol levels in the luteal phase one year after stopping Lupron subcutaneously for 6 months. In 70 patients, the average baseline estradiol level was 137 pg/mL and at one year after stopping Lupron it had miraculously returned to exactly 137, which is literally unbelievable since the same study found that 2 patients (3%) had menopausal estradiol levels (12 pg/mL) at followup . The data table which allegedly supported this miraculous conclusion was not supplied by the drug manufacturer as part of discovery. So at first glance, it appears that the followup level of 137 was fabricated and the tabulation of data supporting this level of 137 was withheld from discovery because the table probably shows that estradiol did not return to baseline in most and the fabricated final number of 137 might be caught. I'm sorry to be so accusatory. I am uncertain if there is a journal publication coming out of M86-050.
5. Fast-forward to 2018. Gallagher et al. (J Pediatr Adolesc Gyn 2018;31:376-81) found that among patients who received Lupron as teenagers and who answered a questionnaire 4 or more years after stopping Lupron, about 45% had symptoms that they considered to be irreversible and due to Lupron. That's not far off from the percentages in paragraphs 2 and 3 above. Most of these side effects were menopausal in character, suggesting low estrogen levels. Rechecking estradiol levels was not part of that study. The authors noted that the Lupron label implied that ovarian function returned to normal by 4 - 6 months after stopping Lupron and were perplexed by the disparity between the label and what they observed in patients. How is this surprising % complaining of symptoms related to low estrogen as identified over 30 years ago?
Simplifying and summarizing 2 - 5 above: 40% of patients have a 40% decline in estradiol from baseline and may not regain normal ovarian function. This risk is not warned of adequately and more patients are being harmed by this drug every week. This risk may persist at the same level for at least four years after stopping Lupron.
6. The FDA's database (AERS) of adverse event reports contains >10,000 reports of adverse events in patients who received Lupron for endometriosis. I wonder how many of those reports suggest low estrogen levels.
DISCUSSION
In M92-878, most of the estradiol levels at baseline were below 60 pg/mL, suggesting that the baseline estradiol was early in an ovarian hormonal cycle when the estradiol level can be low. In other words, around day 3, or sometime soon after menses. In the chart of Lupron-only patients, notice that three of the baseline estradiol levels were over 150, suggesting that these were drawn around mid-cycle when the estradiol level normally ranges between 100 and 300 pg/mL, so perhaps these 3 are oranges being compared to apples. The followup estradiol levels were to be drawn soon after the first vaginal bleeding resembling a menstrual flow had occurred after stopping Lupron, so again early in the cycle. If we discard the 3 baseline values over 150 as being non-comparable, that leaves 13/19 patients (68%) who had low, early cycle estradiol levels at baseline that were pushed even lower by Lupron at followup. So while it is accurate to say that 73% of the entire group of 22 had lower estradiol levels at followup, if only those 13 patients with estradiol levels below 100 at baseline are examined, 13/19 (68%) of patients had lower estradiol levels at followup compared to baseline. This is not a therapeutic coup. The graph below speaks to the entire group of 22 patients.
In M84-042, most of the baseline estradiol levels were above 110 pg/mL, so these were drawn mid-cycle when the estradiol level ranges between 100 and 300. One patient had a low E2 at baseline (45.7) so perhaps one orange being compared to 7 apples. Another patient had a followup estradiol level drawn out of protocol and the original chart in the in-house study was populated using that protocol violation. The chart in my post is populated with the correct estradiol level drawn per protocol. While it is accurate to state that 63% of patients had followup E2 levels lower than baseline, if we discard the single patient with the low baseline level, 6 of 7 (86%!!) had followup E2 lower than baseline at one year after stopping Lupron. The graph below speaks to the entire group of 8 patients.
One of the obvious things that pops out is the small number of patients studied. If 10% of biologic females have endometriosis, that means there are tens of millions of patients with endometriosis. I have no idea how many prescriptions for Lupron have been written over the last 30 years, but let's say 1,000,000. So looking at 22/1,000,000 or 8/1,000,000 may not give an accurate picture of estradiol production after stopping Lupron. But that is a central point: we do not know and so patients cannot make an informed decision. Since this is the only data (suppressed though it was) that exists, it must be taken at face value: most patients do not regain baseline E2 no matter when during the hormonal cycle the E2 is measured and at doses of 3.75 mg/month x 6 months or nasal Lupron x 1 year. Critics might say "The numbers are too small to come to the conclusion you have." The numbers would therefore be too small to come to the conclusion that estradiol production returns to normal in the vast majority of patients receiving Lupron. No question that small numbers can skew results wildly. Is this analysis off by a factor of 2? If so, then 20% of patients may have long-term ovarian dysfunction. Is it off by a factor of 4? If so, then 10% of patients may have long-term ovarian dysfunction. We just don't know the real numbers. The results at least suggest that more study was needed to flesh out this worrisome finding of reduced followup E2 levels, but more study did not occur. Perhaps if the estradiol data one year after stopping Lupron had been included in the published paper, an alarm might have been raised sooner and 3 tragic, wasted, expensive decades - surely one of the darkest eras in American medicine - would not have occurred.
Another thing that pops out is that even back in 1984 there were more sophisticated tests that could be run to check ovarian function. Checking only E2 values is rather crude. But again, it's the only evidence available and has to be taken at face value. The manufacturer can be faulted for studying such small numbers of patients with such crude tests. All of this cries out for more study 30 years on. But the time for more studies to save Lupron has expired. It was irresponsible for the manufacturer to leave this important question hanging and to hide the truth from physicians and patients. While my discussion focuses on the evidence that ovarian function as reflected by estrogen production is damaged long-term by Lupron, GnRH receptors are all over the body and it is possible that other unknown deleterious effects occur by other pathways, possibly including damage to kisspeptin neurons in the hypothalamus. There may be other endocrine disruption beyond ovarian function. In fact, in the publication flowing from M84-048, an effect on the adrenal glands was postulated.
CONCLUSIONS FROM THIS EVIDENCE:
1. Estradiol is very low during Lupron therapy.
2. Estradiol has not returned to baseline in most patients by two to six months after stopping Lupron.
3. Estradiol has not returned to baseline in most patients by one year after stopping Lupron.
4. From another study the manufacturer reported an estradiol recovery at one year after stopping Lupron which is literally unbelievable and did not produce the tabulated data supporting this miraculous recovery. This number appears fabricated.
5. Persistence of hypoestrogenic symptoms in 45% of patients four years or more after stopping Lupron suggests that estradiol may remain depressed permanently in a significant percent of patients.
6. Reports to the FDA of symptoms suggestive of low estradiol levels following Lupron number in the thousands and provide real world support to the conclusion that Lupron seems to damage ovarian function in a substantial fraction of patients taking the drug.
7. Lupron is associated with an unacceptably high risk of ovarian dysfunction or premature ovarian failure and the manufacturer has not produced any evidence to the contrary.
8. It is inconceivable 3 decades on that we still don't know the full picture on ovarian function after Lupron.
9. The substantial risk of possible ovarian dysfunction long after Lupron is discontinued is not mentioned in the product label, as noted by Gallagher et al.
10. Long-term ovarian dysfunction or premature ovarian failure would be a possible explanation for the following statement in a medical publication:
"Many patients benefited from LA (leuprolide acetate, i.e., Lupron) treatment long after the time of exposure." (Dlugi et al Fertil Steril 1990;54:419) Thus, possible ovarian failure has been turned into therapeutic success.
Halle - effin - lujah!
In contrast, the rate of premature ovarian failure after bilateral ovarian cystectomy for bilateral ovarian endometriomas is 2.5%. (Busacca M et al Am J Obstet Gynecol 2006;195:421-5.). So Lupron therapy for women with NORMAL ovaries is 10 - 20 times more injurious to ovarian function than bilateral ovarian cystectomy in women with endometrioma cysts! Just another reason to rush to surgery.
Of course, with just 8 patients having one-year followup of estradiol levels, the manufacturer could say, "8 patients is too few to conclude that the estradiol level is adversely affected by Lupron in a substantial fraction." That cynical self-serving statement would be true at one level. But the correct conclusions would be that the evidence shows that the estradiol level is lower in most patients by one year after stopping Lupron and that more evidence is needed to settle the issue. Other than the fabricated followup estradiol level from M86-050, that evidence has not been produced. I suspect it exists somewhere and was not produced in discovery in Klein v Abbott. After all, we're talking about a drug company here. Marcia Angell, former editor-in-chief of the New England Journal of Medicine has stated: "It is simply no longer possible to believe much of the clinical research that is published." John Gueregian, former Medical Officer for the FDA has said "Lupron causes serious permanent side effects." So it's not just me picking a bone with Lupron.
For 30 years the manufacturer of Lupron has hidden the facts that Lupron therapy is followed by ovarian dysfunction or ovarian failure in a substantial fraction of those receiving it and that it has no evidence in thousands of pages of in-house studies that ovarian function recovers in that fraction. Physicians are unaware of this fact and so cannot obtain informed consent from any patient for this reason. Plus, it is ineffective for pain since the in-house studies showed that about half of patients continued to require narcotics after the initial symptom flare. Just sayin'.
11/20/19: I've created a bar chart and candlestick chart to illustrate more clearly the findings shown in the tables. On the bar chart with the blue ramped background, the bar on the far right represents patients who were exposed to Lupron as teens and who were answering a questionnaire 4 or more years since stopping Lupron and who had symptoms suggestive of low estrogen levels which were thought to be irreversible and dated from Lupron therapy. This serves as a proxy for followup estradiol level lower than baseline, allowing rough comparison with the precding bars. There were a few more symptoms that I didn't include which still could be related to low estrogen levels. Is there any relation of this recent finding to the findings in M84-048?
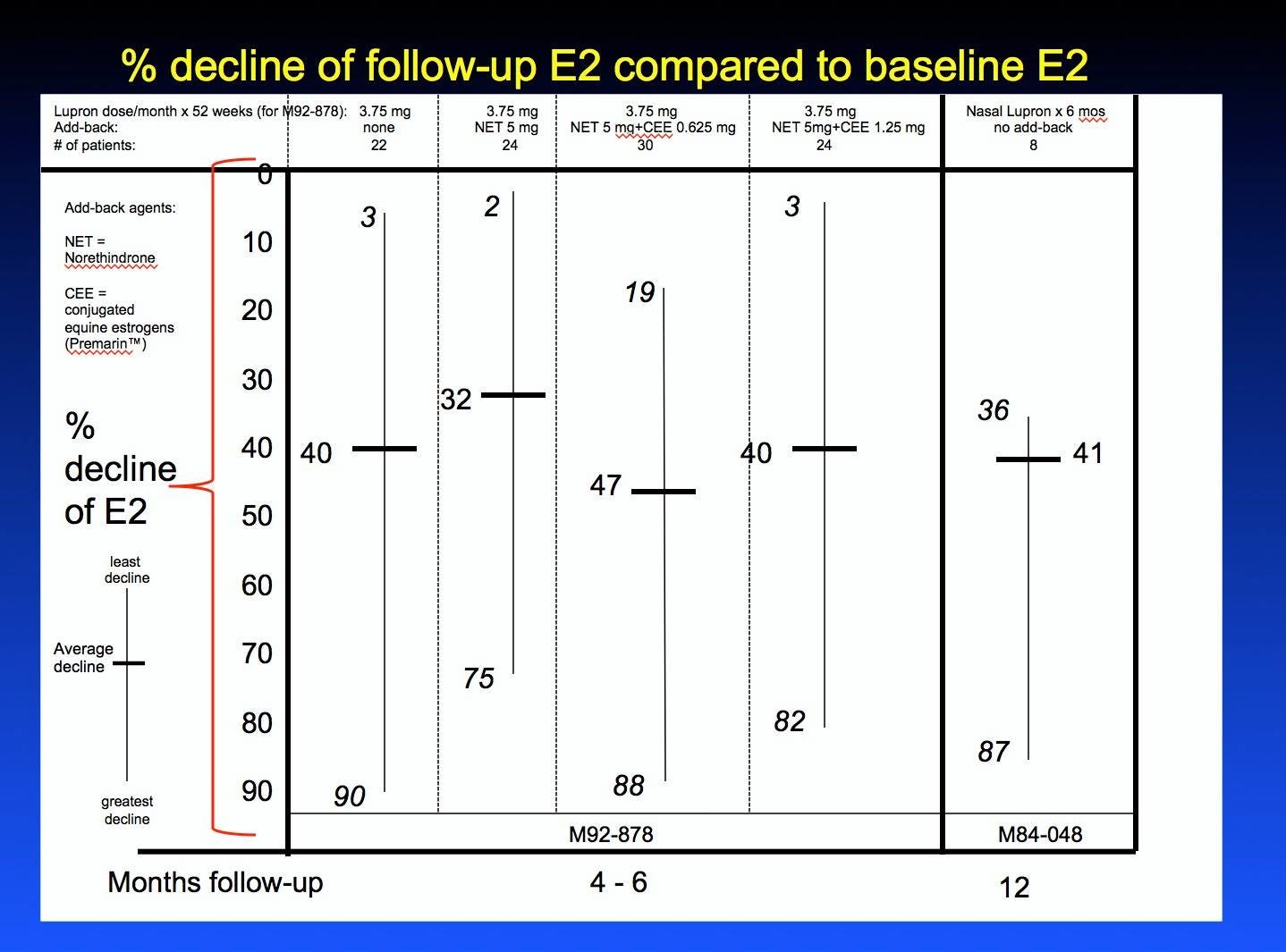
The candlestick chart shows the % reduction of estradiol levels compared to baseline in the two studies (M92-878 at 4 to 6 months after stopping Lupron; M84-048 at 12 months after stopping Lupron) that checked estradiol levels. The numbers next to the short bold horizontal lines show the average % reduction of estradiol from baseline for each of the 4 arms of the study while the range of % reduction is shown by the top and bottom numbers on each line. I didn't put the one year estradiol data from study M86-050 on any chart because the number 137 is likely fabricated.
An obvious trick done with Lupron that was repeated with Orilissa is the simple tactic of just not mentioning poor results in the medical literature. This creates an army of misled physicians who would say "Oh, there's no evidence of that in the literature. (So it must not occur)". With Lupron, it was not mentioning the decline in estrogen and ovulation long after the medicine was stopped. With Orilissa, it was not mentioning the 6 month non-response of painful intercourse, which responded slightly to the higher dose of 200 mg/d at 3 months and so the FDA was suckered into approving Orilissa for the painful sex indication even though the randomized controlled trial that they were considering proved that painful intercourse did not respond to Orilissa. The definition of response in the NEJM paper was taking the medicine for 6 months AND taking the same amount of pain pills OR taking fewer pain pills AND reporting less pain. Since the paper did not mention the 6 month outcome of painful intercourse, it means that painful intercourse did not respond to Orilissa per the protocol definition so the ads and indication are openly deceptive.
Science being controlled by drug companies? What could go wrong?
Note: I never prescribed Lupron during my career. I operated on over 600 patients who had taken Lupron previously who had biopsy-confirmed endometriosis. I unfortunately didn't check hormone levels among those patients, nor did I tabulate complaints that might have indicated low estrogen production. So many patients with endometriosis simply accepted their fates that perhaps they didn't mention estrogen concerns, too. Plus, I, like all the other doctors, thought that these complaints had to be crazy because, well, there was no data to support all this craziness. Sure, Lupron makes you menopausal in the short term, but the effect was temporary. But there appears to be a real possibility that a substantial fraction of patients will not have regained ovarian function long after stopping Lupron. Just looking at the rough numbers on the charts, up to 40% of patients have an average 40% drop in estradiol baseline and ovarian dysfunction can persist in the same % of patients for years after stopping Lupro. Not the sort of effect you'd like to see on young ovaries, especially if you're trying to develop a marketable drug for use in young women in the prime of life. My duty as a physician is to oppose what harms patients. I've chosen this open forum to inform patients directly of my concern for this risk, as bureaucratic wheels grind slowly. The evidence is pretty simple to understand.


Please let this law firm know: https://www.girardsharp.com/work-investigations-puberty-blockers